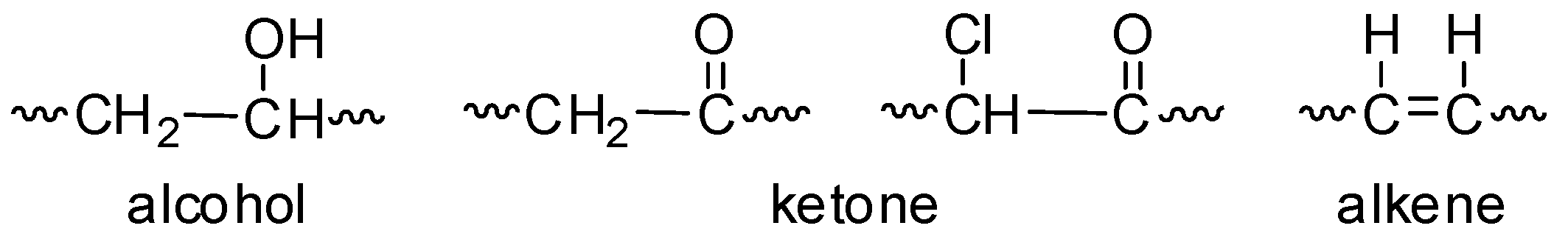
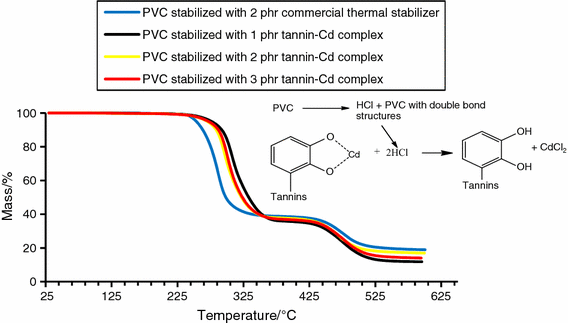
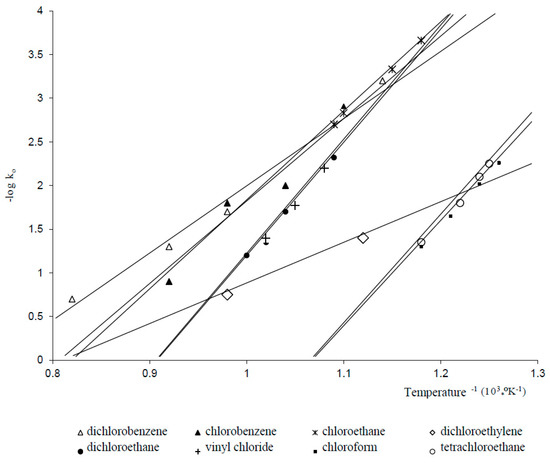
Rate Of Formation Of Vinyl Chloride

Being a very well known memb r of the family of vinyl polymers.
Rate of formation of vinyl chloride. Vinyl chloride is used primarily to make polyvinyl chloride pvc. Vc vc αd 1 vc β βd 2 and vc d 3 were used to study the reactivities of the hydrogen atoms in the polymerization and the β hydrogen atoms contributed to the chain transfer. Polymerization of vinyl chloride vc was studied. What is vinyl chloride.
Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc. Shows the reaction between hydrated electron and vinyl chloride to produce a vinyl radical and a chloride ion koester and asmus 1971 with a rate constant reported as 2 5 10 8 m 1 s 1. With respect to hydrogen chloride satisfactorily describe the rates of transformation of ethylene or vinyl chloride or both in the reactions of oxychlorination of ethylene vinyl chloride and 1 2. Pvc is used to make a variety of plastic products including pipes wire and cable coatings and packaging materials.
About 13 billion kilograms are produced annually. Vinyl chloride is produced from ethylene in two step process. Its annual production rate in the u s a. Vinyl chloride a colourless flammable toxic gas belonging to the family of organohalogen compounds and used principally in making polyvinyl chloride or pvc a widely used plastic with numerous applications.
A rate equation which well represented the data was developed from the following postulates. The purified ethylene dichloride undergoes selective cracking to form vinyl chloride. Natural evolution of hcl from vc occurred in the polymerization. The major industrial preparation of vinyl chloride begins with ethylene and has two.
It does not occur naturally and must be produced industrially for its commercial uses. Chemical and physical methods were used to observe irregular structures such as branching. Has increased at an average annual. The ability of the hydrated electron to degrade vc has also been demonstrated with the ferrous iron uv arp in a previous study liu et al 2013.
Vinyl chloride and incorrectly copolymers containing amow1ts of vinyl idene chloride vinyl acetage ethylene propylene or acrylates. Vinyl chloride is a colorless gas that burns easily. Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.