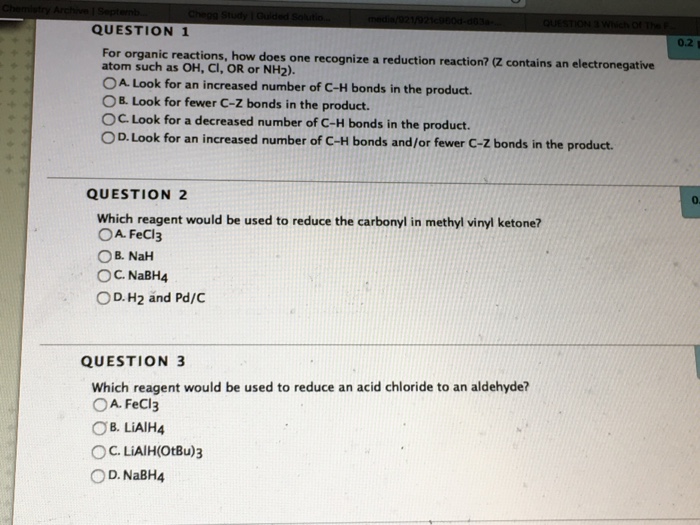
Reagent Reduce Carbonyl In Methyl Vinyl Ketone

Again the product is the same whichever of the two reducing agents you use.
Reagent reduce carbonyl in methyl vinyl ketone. What reagent would be used to reduce an amide to an amine. Aldehydes and ketones can. Only iii o d. Initially the grignard reagent is added to the weinreb amide which further undergoes hydrolysis to furnish ketone.
Carboxylic acids esters and acid halides can be reduced to either aldehydes or a step further to primary alcohols depending on the strength of the reducing agent. Which reagent can be used to reduce an acid chloride to an aldehyde. A because the grignard reagent will react with the acid and be quenched. The enantioselectivity arises from the preference of the larger carbonyl substitutent for the pseudoequatorial position of the ring.
Why can t you use acidic conditions such as aqueous hydrochloric acid for the addition of a grignard reagent to a ketone. Which reagent would be used to reduce the carbonyl in methyl vinyl ketone. Only 1 o c. Reduction of a ketone leads to a secondary alcohol.
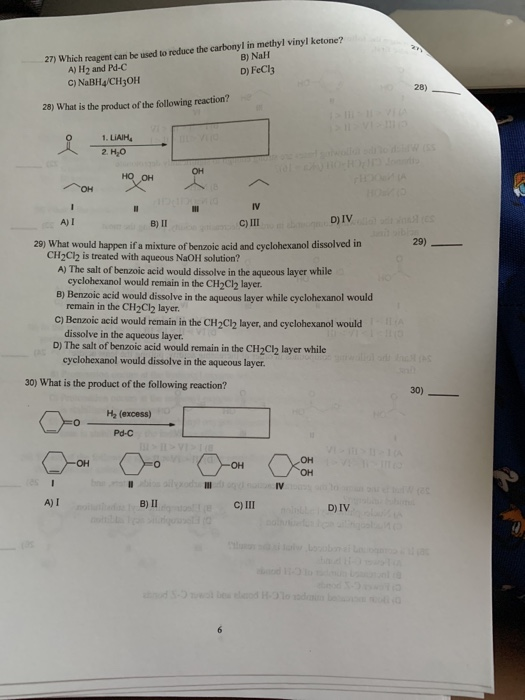
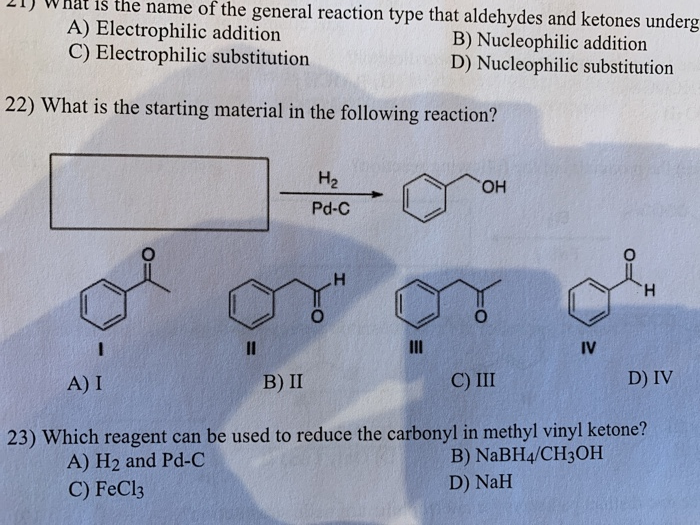
A nabh 4 b lialh otbu 3 c lialh 4 d fecl 3 ans. Nah what is the product of the following reaction. Typical carbonyl compounds are ketones aldehydes carboxylic acids esters and acid halides. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone.
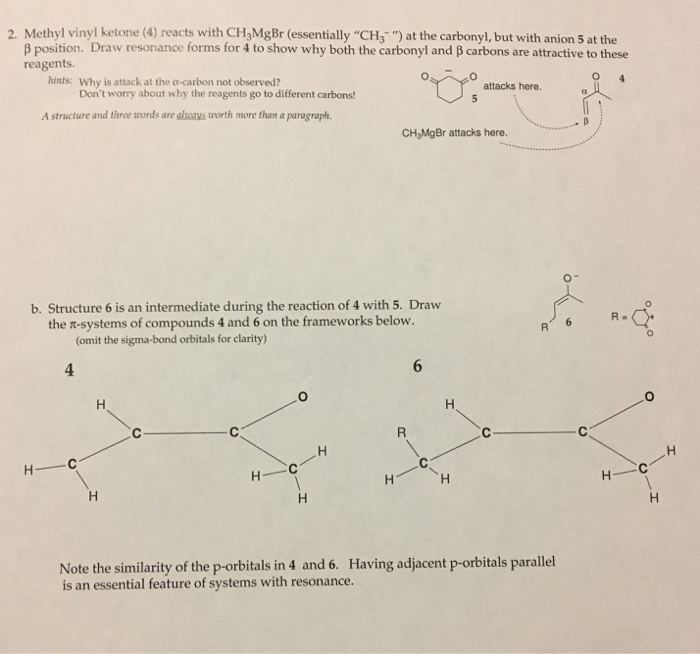
9 addition of an n methoxy n methyl amide also known as weinreb amide rcon me ome to the grignard reagent gives a ketone. Using lithium tetrahydridoaluminate lithium aluminium hydride. For example with propanone you get propan 2 ol. However alkyllithium reagents are less likely to reduce the ketone and may be used to synthesize substituted alcohols.
A nabh4 ch3oh b h2 and pd c c fecl3 d nah. Oh 204 iii select one. B nah d fecl3 27 which reagent can be used to reduce the carbonyl in methyl vinyl ketone. The reduction of a ketone.
When the ketone is sterically hindered using grignard reagents often leads to reduction of the carbonyl group instead of addition. B because the ketone. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone. The less substituted carbon of oxirane is substituted by the alkyl group of grignard reagent.
A h2 and pd c c nabh4 ch3oh 28 28 what is the product of the following reaction. Liam a i 8 ii c iii d iv 29 what would happen if a mixture of benzoic acid and cyclohexanol dissolved in ch2cl2 is treated with aqueous naoh solution. The hydride itself is delivered via a six membered cyclic transition state. H2 and pd c o d.
Only 1 and 11 o b. Below is an example of ethyllithium addition to adamantone to produce tertiary alcohol. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone. In organic chemistry carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.