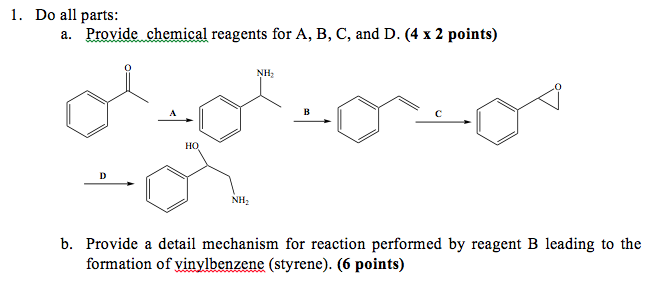
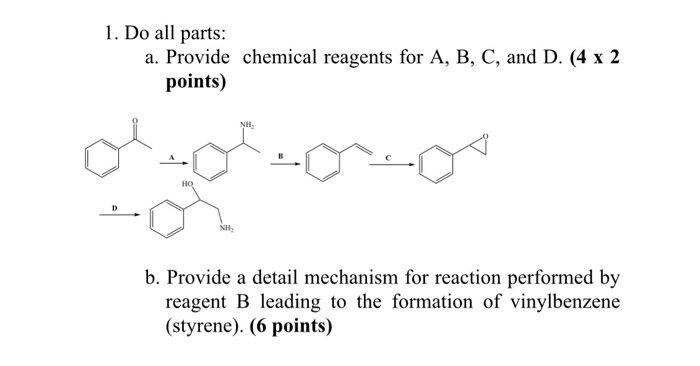
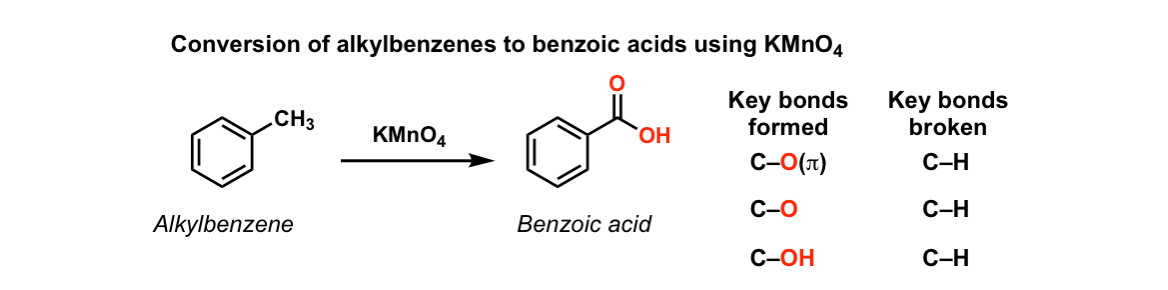
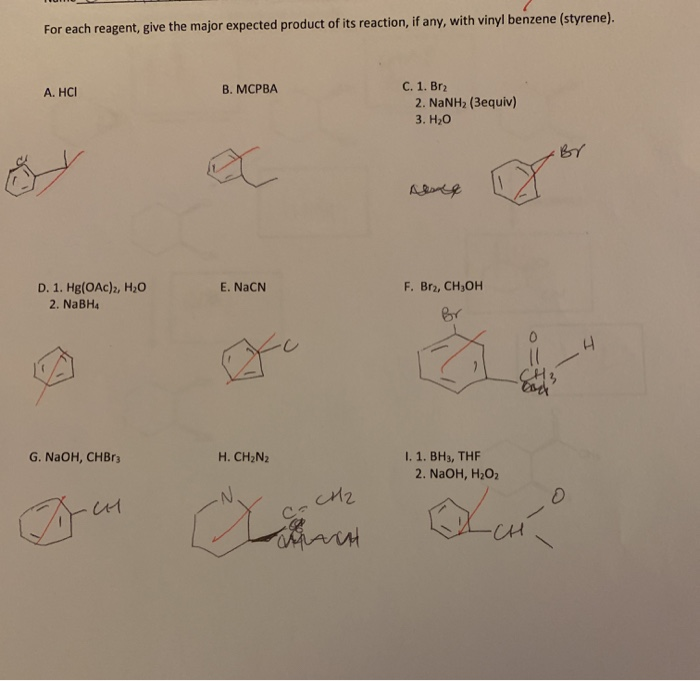
Reagent To Make Vinyl Benzene

And i ll make this one r double prime to distinguish it from the r group on our grignard reagent.
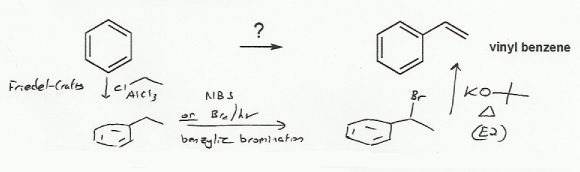
Reagent to make vinyl benzene. In this reaction methylbenzene toluene undergo oxidation process with the reagent of chromyl chloride cro 2 cl 2 present in solution form in ccl 4 or in cs 2 thereby forming chromium complex. And one approach that you can use is the concept of retrosynthesis. An acyl group is an alkyl group attached to a carbon oxygen double bond. The most commonly used acyl group is ch 3 co.
So there is my grignard reagent and i m going to react it with an ester this time. Substitution reactions of benzene and other aromatic compounds. C8h9br koh ethanol c8h8 kbr h2o. Acylation means substituting an acyl group into something in this case into a benzene ring.
The chromium complex undergoes hydrolysis to produce benzaldehyde. And the second step once again we add h3o. We refer to this reaction as etard reaction. So i ll make this r prime.
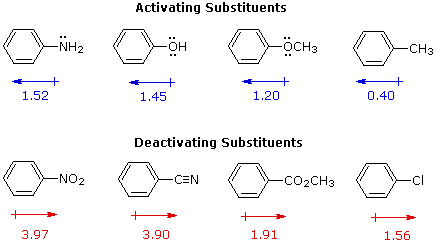
Notice that either of the oxygens can accept the electron pair step 3 loss of a proton from the carbocation to give a new aromatic compound. Well to do that we have to analyze the groups that are attached to our ring. By adding some koh in a dehydrohalogenation reaction. The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier section the chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene.
If r represents any alkyl group then an acyl group has the formula rco. And so our goal is to make this molecule from benzene. Friedel crafts acylation of benzene. If you are carrying this out practically t butoxide is probably a better base to use.
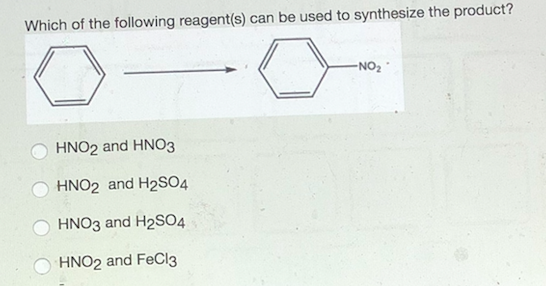
In this situation the grignard reagent is going to add on twice to our carbonyl carbon. In this video i will be synthesizing benzene from sodium benzoate and sodium hydroxide. So you try to think backwards and you think to yourself what can be an immediate precursor to this molecule. Nitration is the usual way that nitro groups are introduced into aromatic rings.