Reduce Carbonyl To Methyl Vinyl Ketone

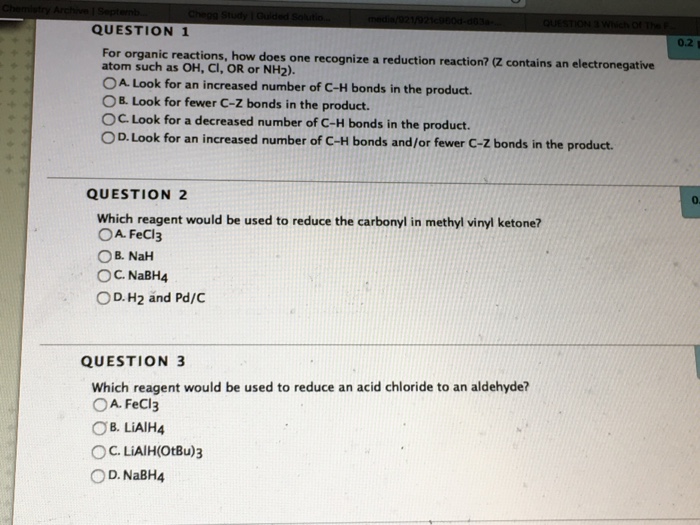
Which reagent can be used to reduce an acid chloride to an aldehyde.
Reduce carbonyl to methyl vinyl ketone. Oh 204 iii select one. Directly at the carbonyl c or the end of the conjugated system. In organic chemistry carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent. H2o oh oh но.
The bimolecular reactions of methyl vinyl ketone oxide mvk oo with h 2 o h 2 s nh 3 their dimer forms and ch 3 nh 2 have been studied the reactions of h 2 o 2 and nh 3 2 exhibit an effect of reducing the free energies of activation δg. Note the red region show the high electron density at the o of the c o and the lower electron density of the conjugated c c compare this with. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone. Liam a i 8 ii c iii d iv 29 what would happen if a mixture of benzoic acid and cyclohexanol dissolved in ch2cl2 is treated with aqueous naoh solution.
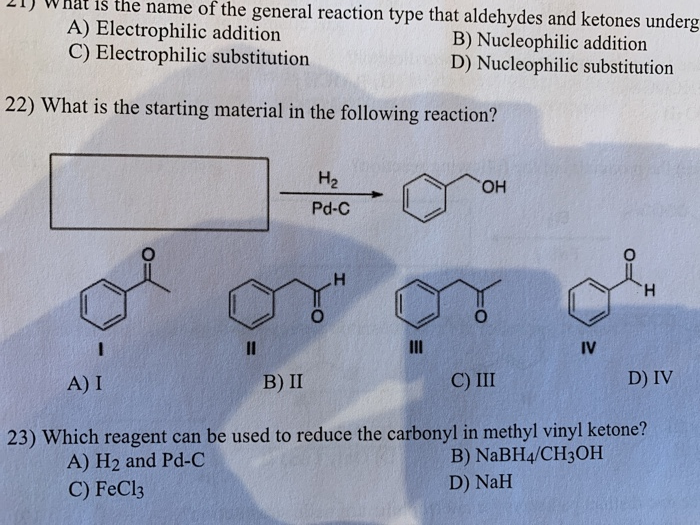
Carbonyl group is a common functional group in organic chemistry with a wide range of reactivity. Which reagent can be used to reduce an acid chloride to an aldehyde. Nah what is the product of the following reaction. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone.
What reagent would be used to reduce an amide to an amine. Carboxylic acids esters and acid halides can be reduced to either aldehydes or a step further to primary alcohols depending on the strength of the reducing agent. Only iii o d. H2 and pd c o d.
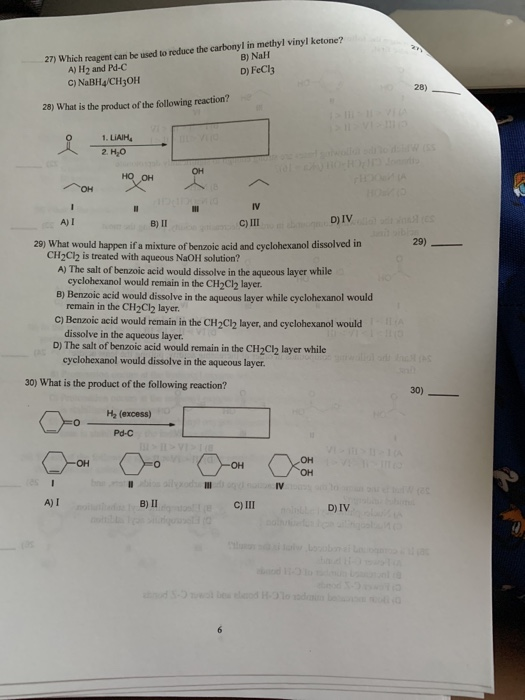
Only 1 o c. A h2 and pd c c nabh4 ch3oh 28 28 what is the product of the following reaction. Reduction of a ketone leads to a secondary alcohol. The reduction of a ketone.
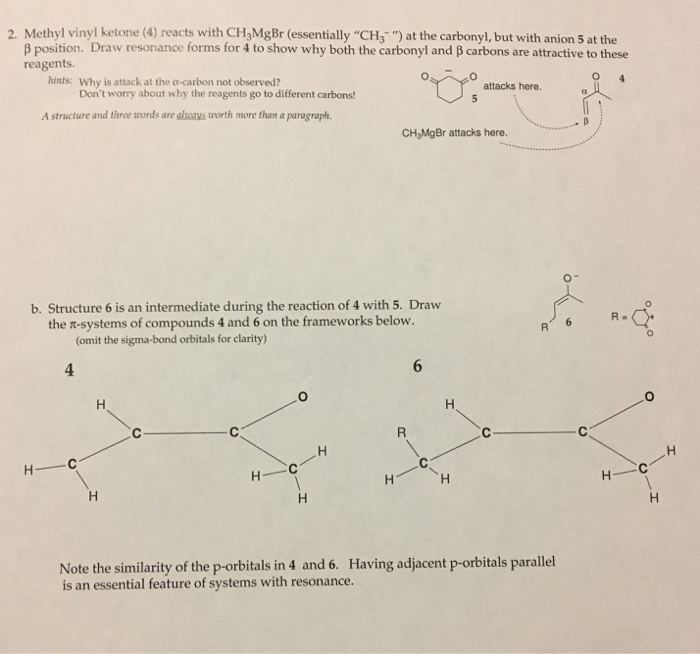
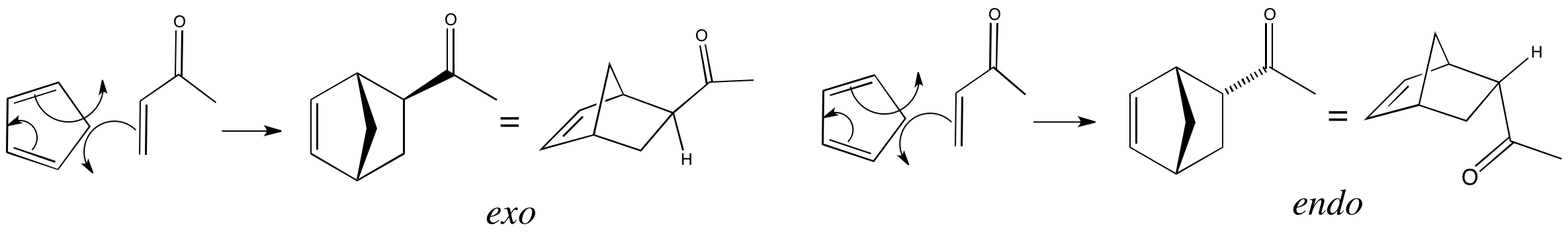
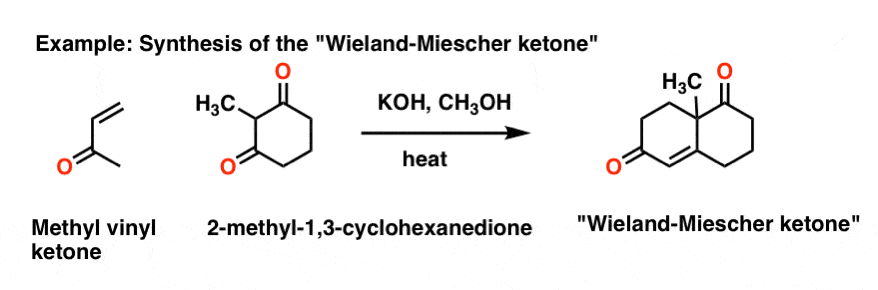
What reagent would be used to reduce an amide to an amine. The simplest enone is methyl vinyl ketone butenone ch 2 chcoch 3 enones are typically produced using an aldol condensation or knoevenagel condensation some commercially significant enones produced by condensations of acetone are mesityl oxide dimer of acetone and phorone and isophorone. Methyl vinyl ketone 1 3 butadiene. The two types of carbonyls we are familiar with are ketone and aldehydes.
Only 1 and 11 o b. A nabh4 ch3oh b h2 and pd c c fecl3 d nah. Again the product is the same whichever of the two reducing agents you use. For example with propanone you get propan 2 ol.
Using lithium tetrahydridoaluminate lithium aluminium hydride. The key difference between carbonyl and ketone is that all carbonyl groups have a carbon atom with double bonded oxygen atom whereas the ketones have a carbonyl group attached to two alkyl groups. An enone is a type of an α β unsaturated carbonyl that consists of an alkene conjugated to a ketone. A nabh4 b lialh otbu 3 c lialh4 d fecl3.
H2 and pd c c. Which reagent can be used to reduce the carbonyl in methyl vinyl ketone. A catalytic behavior the former energy barriers decrease by 20 kj mol 1 while the latter by 14 kj mol 1.